en-uklanguageSelector_en-uk
en-uklanguageSelector_en-ukStaying safe in the Internet of Medical Things (IoMT)
Software and hardware developed by experts
tuomi has been developing medical software products that comply to the European Medical Device Regulation (EU MDR) since 2016. We offer software services for cloud and mobile applications as well as engineering for medical hardware.
Our engineering and development always fulfils all relevant standards, including:
Medical Device Regulation 2017/745/EU
ISO 14971:2019: Medical devices - Application of risk management to medical devices
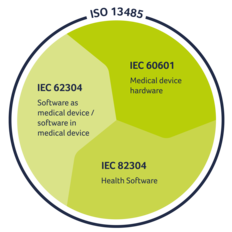
EN 62304:2006 + Cor.:2008 + A1:2015: Medical device software - Software life cycle process
- IEC
62366-1:2015 + AC:2015: Medical devices
IEC 82304-1: Healthcare software
Expert advise
We do more than just provide support and expert advice. We always work closely with you to implement your specific medical project. And after 5 years of experience with the new EU Medical Device Regulation, our customers and partners know they can rely on a team that has a lot of experience with the EU MDR environment.
QMS
We are only ever satisfied with the highest quality, so that is exactly what we offer our partners and customers. The high quality is guaranteed through the processes and goals we have established within the company.
Blog

AI in Medicine: Human Expertise Remains Indispensable
Artificial intelligence (AI) is rapidly reshaping both the world of work and the healthcare sector. A frequently cited PwC study suggests that AI could boost global GDP by up to 15% by 2035. In medicine, expectations are high: AI systems promise to reduce the workload for healthcare professionals, streamline processes, and offer valuable decision support. Yet one thing remains clear: technological progress cannot replace human expertise.
tuomi at medtech 2025
Under the motto: "With digital medical technology and data-driven solutions toward the connected healthcare system of the future: How medical technology is reinventing itself through impulses from design and digital technologies."
Dr Enise Lauterbach becomes tuomis new Medical Director
Dr Enise Lauterbach, a well-known German doctor and award-winning founder, joins the IT specialist tuomi. As Medical Director, she will take on an advisory role and support tuomi in developing further highly specialised medical software for medical staff and patients.